Introduction : Chimeric antigen receptor T (CAR T) is an effective therapy for refractory/relapsed (R/R) philadelphia chromosome positive acute lymphoblastic leukemia (ph+ ALL) patients in TKIs era. Patients with lymphoid blastic phase of chronic myeloid leukemia (CML-LBC) also have BCR-ABL fusion oncogenes, and usually shows an aggressive clinical course with a poor prognosis. However, outcomes of CAR T therapy for patients with R/R CML-LBC is still uncertain. This study was designed to compare the efficacy and safety of CAR T-cells therapy in patients with R/R CML-LBC and ph+ ALL.
Patients and Methods : Clinical data from 13 patients with R/R CML-LBC and 121 R/R ph+ ALL patients who received CAR T treatments at our center from February 2017 to March 2023 were retrospectively collected and analyzed. Before CAR T-cells treatment, fifty patients (11.2%) had primary refractory disease, 106 patients (79.1%) were experiencing the first relapse, and 13 patients (9.7%) were experiencing the second or more relapse. All patients received lymphodepletion with fludarabine (30 mg/m2 /d) and cyclophosphamide (300 mg/m2 /d) -based conditioning regimens on day −5 to −3. CAR T-cells were then infused at day 0. Among them, 8 patients with CML-LBC and 92 ph+ ALL patients received single CD19 CAR T therapy, others received tandem CD19/CD22 CAR T.
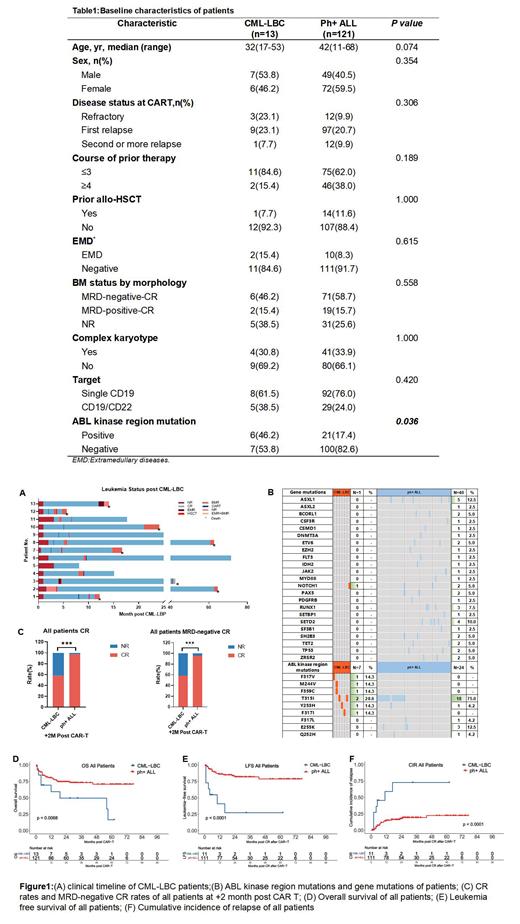
Results:The baseline characteristics of all patients were shown in Table 1. Patients with CML-LBC carried a higher proportion of mutations in the ABL kinase region ( P=0.036).The clinical timeline of CML-LBC patients as shown in Figure 1A,and ABL kinase region mutations and gene mutations of patients are shown in Figure 1B. Patients with CML-LBC (58.3%) had significantly lower CR rates than ph+ ALL (97.2%) patients at +2 month after CAR-T therapy (P<0.001) ( Fig. 1B). ABL kinase region mutation (odds ratio, 0.091; 95% CI, 0.015-0.571) and CML-LBC (odds ratio, 0.063; 95% CI, 0.010-0.376) remained risk factors in multivariate logistic regression analysis of CR rate in all patients. Furthermore, minimal residual disease (MRD)-negative CR rates were 58.3% and 95.3% at +2 month after CAR T-cells therapy, respectively (P<0.001) ( Fig. 1B). There was no significant difference in the incidence of adverse events among the two groups. Severe cytokine release syndrome (CRS, Grade ≥ 3) occurred in 7.7% of patients with CML-LBC and 8.3% in ph+ ALL patients (P=1.000). Moreover, patients with CML-LBC showed poorer 2-year prognoses than the ph+ ALL patients (overall survival: P=0.0068; leukemia-free survival: P<0.0001; cumulative incidence of relapse: P<0.001) ( Fig. 1C-1E). Univariate and multivariate Cox regression analyses showed that a poorer LFS was related to ABL kinase region mutation (hazard ratio, 3.257; 95% CI, 1.404-7.553) and CML-LB (hazard ratio, 3.803; 95% CI, 1.507-9.596).
Conclusions: Worser sustained antitumor efficacy and long-term survival with CAR T-cells therapy in patients with CML-LBC compared to ph+ ALL patients, and ABL kinase region mutation is an independent risk factor for CR and LFS.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal